
CAS number: 59-02-9
Molecular formula: C29H50O2
molecular weight: 430.7061
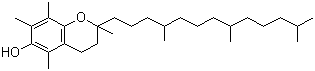
Chemical structure:
| Product Name | Vitamin E Oil | |
| Shelf Life | 3 years | |
| Item | Specification | Result |
| Description | Clear, colourless slightly greenish-yellow, viscous, oily liquid, EP/USP/FCC | Clear, slightly greenish-yellow, Viscous, oily liquid |
| Identification | ||
| A Optical rotation | -0.01° to +0.01°, EP | 0.00° |
| B IR | To conform, EP/USP/FCC | conform |
| C Color reaction | To conform, USP/FCC | conform |
| D Retention time, GC | To conform, USP/FCC | conform |
| Related Substances | ||
| Impurity A | ≤5.0%, EP | <0.1% |
| Impurity B | ≤1.5%, EP | 0.44% |
| Impurity C | ≤0.5%, EP | <0.1% |
| Impurity D and E | ≤1.0%, EP | <0.1% |
| Any other impurity | ≤0.25%, EP | <0.1% |
| Total impurities | ≤2.5%, EP | 0.44% |
| Acidity | ≤1.0ml, USP/FCC | 0.05mL |
| Residual Solvents (Isobutyl acetate) | ≤0.5%, In-house | <0.01% |
| Heavy metals (Pb) | ≤2mg/kg,FCC | <0.05mg/kg(BLD) |
| Arsenic | ≤1mg/kg,In-house | <1mg/kg |
| Copper | ≤25mg/kg,In-house | <0.5m/kg(BLD) |
| Zinc | ≤25mg/kg,In-house | <0.5m/kg(BLD) |
| Assay | 96.5% to 102.0%, EP 96.0% to 102.0%, USP/FCC | 99.0%, EP 99.0%, USP/FCC |
| Microbiological tests | ||
| Total aerobic microbial count | ≤1000cfu/g,EP/USP | Certified |
| Total yeasts and moulds count | ≤100cfu/g,EP/USP | Certified |
| Escherichia coli | n.d./g,EP/USP | Certified |
| Salmonella | n.d./g,EP/USP | Certified |
| Pseudomonas aeruginosa | n.d./g,EP/USP | Certified |
| Staphyloscoccus aureus | n.d./g,EP/USP | Certified |
| Bile-Tolerant Gram-Negative Bacteria | n.d./g,EP/USP | Certified |
| Conclusion: Conform to EP/USP/FCC | ||